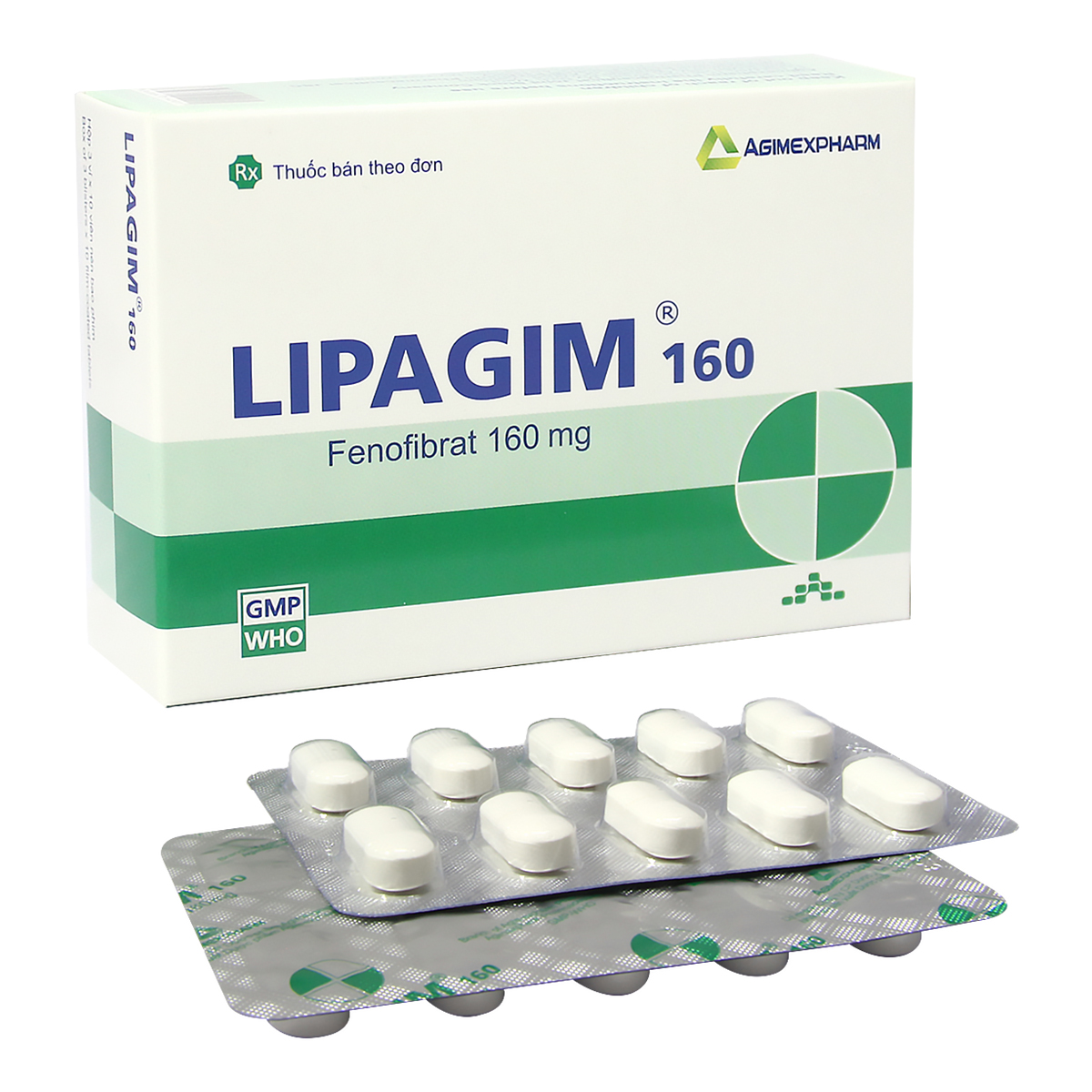
Chỉ định:
Điều trị tăng lipid máu của các typ IIa, IIb, III, IV và V ở bệnh nhân không đáp ứng thỏa đáng với chế độ ăn.
Liều dùng và cách dùng:
Cách dùng:
Phải uống thuốc cùng với bữa ăn và phối hợp với chế độ ăn hạn chế lipid.
Liều dùng:
Người lớn: Liều khuyến cáo là 1 viên, một lần mỗi ngày. Cần duy trì liều ban đầu cho đến khi cholesterol máu trở lại bình thường. Phải kiểm tra cholesterol máu 3 tháng một lần.
Bệnh nhân cao tuổi ( ≥ 65 tuổi): Ở những bệnh nhân lớn tuổi, không có suy thận, dùng theo liều khuyến cáo của người lớn.
Bệnh nhân suy thận: Lipagim 160 không được khuyến cáo dùng cho người suy thận nặng.
Bệnh nhân suy gan: Lipagim 160 không được khuyến cáo dùng cho người suy gan.
Nếu nồng độ lipid máu không giảm nhiều sau 3 đến 6 tháng điều trị bằng fenofibrat thì cần thay đổi trị liệu (trị liệu bổ sung hoặc trị liệu khác).
An toàn và hiệu quả khi sử dụng thuốc cho trẻ em dưới 18 tuổi chưa được xác định rõ, vì vậy không khuyến cáo sử dụng thuốc cho trẻ em dưới 18 tuổi.
Chống chỉ định:
Quá mẫn với bất cứ thành phần nào của thuốc.
Suy thận nặng.
Rối loạn chức năng gan, bao gồm xơ gan ứ mật tiên phát và dai dẳng không rõ nguyên nhân.
Bệnh nhân có bệnh lý túi mật.
Bệnh nhân viêm tụy cấp hoặc mạn tính trừ trường hợp viêm tụy cấp do tăng triglycerid máu nặng, đã có phản ứng dị ứng với ánh sáng khi điều trị với các fibrat hoặc ketoprofen.
Bệnh nhân dùng đồng thời với các fibrat khác.
Trẻ dưới 10 tuổi.
Phụ nữ có thai và cho con bú.
Các trường hợp thận trọng khi dùng thuốc:
Các tình trạng cần thận trọng:
Nhất thiết phải thăm dò chức năng gan và thận của người bệnh trước khi bắt đầu dùng fibrat.
Chức năng gan:
Cũng như những thuốc hạ lipid máu khác, tăng aminotransferase đã được báo cáo ở một số bệnh nhân. Phải theo dõi chức năng gan định kỳ (3 tháng một lần) trong 12 tháng đầu điều trị. Nếu nồng độ aminotransferase huyết thanh (AST, ALT) vượt quá 3 lần mức bình thường hoặc SGPT (ALT) > 100 đơn vị quốc tế thì phải ngừng điều trị fenofibrat.
Không kết hợp fenofibrat với các thuốc có tác dụng độc với gan.
Viêm tụy, sỏi mật:
Cũng như các dẫn chất acid fibric khác, fenofibrat có thể làm tăng bài xuất cholesterol vào mật, dẫn đến bệnh sỏi mật. Nếu kiểm tra túi mật thấy có sỏi thì phải ngừng fenofibrat.
Biến chứng mật dễ xảy ra ở người bệnh xơ ứ gan mật hoặc có sỏi mật.
Đã từng gặp viêm tụy ở người bệnh điều trị bằng fenofibrat hoặc các dẫn chất acid fibric khác.
Viêm cơ, bệnh cơ và/hoặc tiêu cơ vân đã được thông báo ở người bệnh dùng fenofibrat hoặc các dẫn chất acid fibric khác. Tiêu cơ vân và các biến chứng khác cũng đã được thông báo ở người bệnh dùng đồng thời fenofibrat cùng với một số thuốc hạ lipid máu khác như các statin (các chất ức chế HMG – CoA Reductase). Các bệnh nhân dùng fenofibrat phải được hướng dẫn báo cáo ngay khi thấy đau cơ không rõ nguyên nhân, hoặc nhạy cảm đau, yếu, đặc biệt nếu kèm khó thở hoặc sốt.
Phải theo dõi định kỳ enzym creatin kinase (CK hoặc CPK) ở những người bệnh có những tác dụng phụ đó. Phải ngừng điều trị fenofibrat nếu nồng độ CPK huyết thanh tăng cao rõ rệt hoặc nghi ngờ hoặc chẩn đoán là viêm cơ hoặc bệnh cơ.
Nhược năng giáp có thể là một yếu tố làm tăng khả năng bị tác dụng phụ ở cơ.
Chức năng thận:
Fenofibrat chống chỉ định ở bệnh nhân suy thận nặng.
Fenofibrat nên được sử dụng thận trọng ở bệnh nhân suy thận nhẹ đến trung bình. Liều lượng nên được điều chỉnh ở những bệnh nhân có mức lọc cầu thận khoảng 30 – 59 ml/phút/1,73m2.
Khuyến cáo nên đo creatinin trong 3 tháng đầu sau khi bắt đầu điều trị và định kỳ sau đó.
Nguyên nhân gây tăng lipid máu thứ phát:
Nguyên nhân tăng cholesterol máu thứ phát, chẳng hạn như bệnh đái tháo đường typ 2 không được kiểm soát, nhược giáp, hội chứng thận hư, loạn protein huyết, bệnh gan do tắc nghẽn hoặc nghiện rượu nên được điều trị trước khi dùng fenofibrat. Nguyên nhân tăng cholesterol máu thứ phát có liên quan đến điều trị bằng thuốc có thể thấy với các thuốc lợi tiểu, thuốc chẹn β, estrogen, progesteron, thuốc tránh thai kết hợp, thuốc ức chế miễn dịch và các chất ức chế protease. Những trường hợp này cần phải xác định chắc chắn tăng lipid máu nguyên phát hay thứ phát (có thể tăng lipid do những tác nhân điều trị khác).
Thay đổi nồng độ creatinin huyết thanh: Mức gia tăng creatinin huyết thanh đã được báo cáo ở những bệnh nhân dùng fenofibrat. Mức gia tăng này có xu hướng quay trở lại ban đầu sau khi ngừng dùng fenofibrat. Ý nghĩa lâm sàng của sự gia tăng này chưa biết rõ. Do đó khuyến cáo đo creatinin trong 3 tháng đầu tiên sau khi bắt đầu điều trị và định kỳ sau đó. Cũng cần kiểm tra creatinin ở bệnh nhân dùng fenofibrat có nguy cơ bị suy thận như người già và bệnh nhân đái tháo đường. Điều trị nên ngưng khi nồng độ creatinin > 50% trên giới hạn bình thường.
Giảm nghịch lý mức độ HDL Cholesterol (HDL-C): Đã có báo cáo hậu mại và báo cáo thử nghiệm lâm sàng giảm nghiêm trọng mức độ HDL-C (nhỏ nhất là 2 mg/dl) xảy ra ở bệnh nhân đái tháo đường và bệnh nhân không đái tháo đường điều trị với fibrat. Sự giảm HDL-C được biểu hiện bởi giảm apolipoprotein A1. Sự sụt giảm này đã được báo cáo xảy ra trong vòng 2 tuần đến 1 năm sau khi bắt đầu điều trị với fibrat. Mức HDL-C vẫn còn thấp cho đến khi ngừng điều trị fibrat; đáp ứng ngưng điều trị với fibrat nhanh chóng và bền vững. Ý nghĩa lâm sàng của những thay đổi này chưa biết rõ. Do đó khuyến cáo nên kiểm tra mức độ HDL-C trong vòng vài tháng đầu tiên sau khi bắt đầu điều trị với fibrat. Nếu phát hiện mức HDL-C giảm nghiêm trọng, nên ngưng điều trị fibrat, theo dõi mức HDL-C cho đến khi trở về mức ban đầu, không nên dùng lại liệu pháp fibrat.
Thay đổi trong một số xét nghiệm hóa học: Giảm nhẹ và vừa hemoglobin, hematocrit và bạch cầu đã gặp ở người bệnh dùng fenofibrat. Tuy vậy, các thông số này thường trở về bình thường trong quá trình điều trị dài hạn. Đã gặp một số hiếm trường hợp giảm tiểu cầu và mất bạch cầu hạt được báo cáo ở những người dùng fenofibrat. Khuyến cáo kiểm tra định kỳ hồng cầu và bạch cầu trong 12 tháng đầu điều trị với fenofibrat.
Phản ứng quá mẫn: Một số hiếm trường hợp ban da nặng phải nhập viện và dùng liệu pháp corticosteroid, bao gồm hội chứng Stevens-Johnson và hoại tử biểu bì nhiễm độc đã được thông báo ở những người được điều trị bằng fenofibrat. Mày đay và ban da cũng được thông báo ở khoảng 1% người bệnh dùng fenofibrat so với người dùng giả dược trong các thử nghiệm có kiểm soát.
Nếu sau vài tháng dùng thuốc (3 – 6 tháng) mà thấy lượng lipid trong máu thay đổi không đáng kể thì phải xem xét trị liệu khác (bổ sung hoặc khác).
Tính an toàn và hiệu quả của fenofibrat chưa được xác định ở trẻ em dưới 18 tuổi.
Các khuyến cáo dùng thuốc cho phụ nữ có thai và cho con bú:
Thời kỳ mang thai: Không nên dùng trong thời kỳ mang thai.
Thời kỳ cho con bú: Không có dữ liệu. Tuy nhiên, vì an toàn, không nên dùng cho người cho con bú.
Tác động của thuốc đến khả năng lái xe và vận hành máy móc:
Chưa thấy thuốc có ảnh hưởng đến khả năng lái xe và vận hành máy móc.
Tương tác của thuốc với các thuốc khác và các loại tương tác khác:
Thuốc ức chế HMG CoA reductase và fibrat khác: Dùng kết hợp fibrat với các thuốc ức chế HMG CoA reductase (ví dụ: Pravastatin, simvastatin, fluvastatin) hoặc fibrat khác sẽ làm tăng đáng kể nguy cơ tổn thương cơ.
Cyclosporin: Kết hợp fibrat với cyclosporin làm tăng nguy cơ độc tính thận do cyclosporin.
Thuốc chống đông máu đường uống: Fenofibrat làm tăng tác dụng của các thuốc uống chống đông máu nên làm tăng nguy cơ xuất huyết do đẩy các thuốc này ra khỏi vị trí gắn với protein huyết tương. Phối hợp này không khuyến cáo dùng đồng thời, nếu dùng cần theo dõi lượng prothrombin máu. Khi bắt đầu dùng fibrat, cần giảm liều thuốc chống đông xuống chỉ còn 1/3 liều cũ và điều chỉnh liều thuốc chống đông trong quá trình dùng theo INR (International Normalised Ratio) và sau khi ngừng dùng fibrat 8 ngày.
Glitazones: Một số trường hợp giảm có thể hồi phục của HDL-cholesterol đã được báo cáo trong quá trình dùng đồng thời fenofibrat và glitazons. Do đó, khuyến khích kiểm tra HDL-cholesterol nếu phối hợp hai thuốc này và ngưng dùng một trong hai thuốc nếu HDL-cholesterol quá thấp.
Các thuốc chuyển hóa qua CYP2C19, CYP2A6 và CYP2C9:
Nghiên cứu in vitro trên microsome gan người cho thấy fenofibrat và acid fenofibric không ức chế cytochrom (CYP) P450, chất đồng dạng CYP3A4, CYP2D6, CYP2E1 hoặc CYP1A2. Thuốc này là những chất ức chế yếu của CYP2C19 và CYP2A6 và chất ức chế nhẹ đến trung bình của CYP2C9 ở nồng độ điều trị.
Bệnh nhân dùng đồng thời fenofibrat và các thuốc chuyển hóa qua CYP2C19, CYP2A6 và đặc biệt là CYP2C9 có chỉ số điều trị hẹp nên cần được theo dõi cẩn thận và nếu cần thiết, khuyến cáo điều chỉnh liều của các thuốc này.
Không được dùng kết hợp các thuốc độc với gan (thuốc ức chế MAO, perhexilin maleat …) với fenofibrat.
Tác dụng không mong muốn:
Tác dụng không mong muốn thường nhẹ và ít gặp.
Thường gặp, ADR > 1/100:
Tiêu hóa: Rối loạn dạ dày hoặc ruột (đau bụng, táo bón, buồn nôn).
Gan: Tăng aminotransferase (liên quan đến liều).
Cơ xương: Đau lưng, tăng CPK.
Hô hấp: Bệnh hô hấp, viêm mũi.
Ít gặp, 1/1000 < ADR < 1/100:
Hệ thần kinh TW: Nhức đầu.
Da: Nổi ban, nổi mày đay, ban không đặc hiệu.
Cơ xương: Rối loạn cơ bắp (ví dụ như đau cơ, viêm cơ, co thắt cơ bắp và yếu).
Máu: Creatinin huyết thanh tăng.
Hiếm gặp, ADR < 1/1000:
Gan: Sỏi đường mật.
Sinh dục: Mất dục tính và liệt dương, giảm tinh trùng.
Máu: Giảm bạch cầu, giảm hemoglobin.
Rối loạn hệ thống miễn dịch: Quá mẫn.
Da và các rối loạn mô dưới da: Phản ứng với ánh sáng.
Tần số chưa biết (không thể được ước tính từ dữ liệu có sẵn):
Rối loạn gan mật: Vàng da, biến chứng của sỏi mật (ví dụ như viêm túi mật, viêm đường mật, cơn sỏi mật).
Da và các rối loạn mô dưới da: Phản ứng da nghiêm trọng (ví dụ như hồng ban đa dạng, hội chứng Stevens-Johnson, hoại tử biểu bì độc hại).
Rối loạn cơ: Tiêu cơ vân.
Hướng dẫn cách xử trí ADR: Tạm ngừng dùng thuốc.
Quá liều và cách xử trí:
Triệu chứng: Không có trường hợp quá liều đã được báo cáo.
Xử trí: Nếu có trường hợp nghi ngờ quá liều, điều trị triệu chứng và dùng các biện pháp hỗ trợ thích hợp như gây nôn hoặc rửa dạ dày. Thẩm tách máu không có tác dụng loại bỏ fenofibrat khỏi cơ thể.
Các đặc tính dược lực học:
Fenofibrat, dẫn chất của acid fibric, là thuốc hạ lipid máu, tác dụng này được thực hiện qua trung gian chất hoạt hóa Peroxisome Proliferator Activated Receptor type alpha (PPARα).
Thông qua chất hoạt hóa PPARα, fenofibrat làm tăng lipolysis và loại bỏ các thành phần gây xơ vữa giàu triglycerid từ huyết tương bằng cách kích hoạt lipoprotein lipase và giảm sản xuất apoprotein CIII (chất ức chế hoạt động lipoprotein lipase). Kích hoạt của PPARα cũng gây ra một sự gia tăng trong việc tổng hợp apoprotein AI và AII.
Các tác dụng nêu trên của fenofibrat trên lipoprotein dẫn đến giảm lipoprotein tỷ trọng rất thấp (VLDL) và thấp (LDL) có chứa apoprotein B và tăng phần lipoprotein tỷ trọng cao (HDL) có chứa apoprotein AI và AII.
Mối liên quan giữa tăng cholesterol máu và vữa xơ động mạch đã được xác lập, và cả mối liên quan giữa vữa xơ động mạch và nguy cơ mạch vành. Nồng độ HDL hạ có liên quan đến nguy cơ mạch vành cao. Nồng độ triglycerid cao cũng có liên quan đến tăng nguy cơ tim mạch.
Trong các thử nghiệm lâm sàng, fenofibrat có thể làm giảm nồng độ cholesterol trong máu từ 20-25%, triglycerid từ 40-55% và HDL cholesterol đã tăng từ 10-30%.
Do ảnh hưởng quan trọng của fenofibrat đối với cholesterol LDL và triglycerid, điều trị với fenofibrat có lợi ở những bệnh nhân tăng cholesterol máu có hoặc không có tăng triglycerid máu, bao gồm tăng lipoprotein máu thứ phát ở bệnh nhân đái tháo đường typ 2.
Fenofibrat được dùng để điều trị tăng lipoprotein – huyết các typ IIa, IIb, III, IV và V cùng với một chế độ ăn rất hạn chế về lipid.
Fenofibrat cũng làm giảm acid uric máu ở người bình thường và người tăng acid uric máu do làm tăng đào thải acid uric ra nước tiểu.
Các đặc tính dược động học:
Hấp thu: Fenofibrat được hấp thu ngay ở đường tiêu hóa khi uống cùng với thức ăn. Hấp thu của fenofibrat tăng lên khi dùng chung với thức ăn. Nồng độ đỉnh (Cmax) trong huyết tương xuất hiện khoảng 5 giờ sau khi uống thuốc.
Chuyển hóa và phân bố: Thuốc nhanh chóng thủy phân thành acid fenofibric là chất có hoạt tính, chất này được liên kết mạnh với albumin huyết tương (hơn 99%).
Đào thải: Acid fenofibric đào thải chủ yếu theo nước tiểu (70% trong vòng 24 giờ), chủ yếu dưới dạng liên hợp glucuronic, ngoài ra còn có acid fenofibric dưới dạng khử và chất liên hợp glucuronic của nó. Ở người có chức năng thận bình thường, nửa đời trong huyết tương của acid fenofibric vào khoảng 20 giờ nhưng thời gian này tăng lên rất nhiều ở người mắc bệnh thận và acid fenofibric tích luỹ đáng kể ở người bệnh suy thận uống fenofibrat hàng ngày. Hầu hết các sản phẩm được đào thải trong vòng 6 ngày.


 English
English