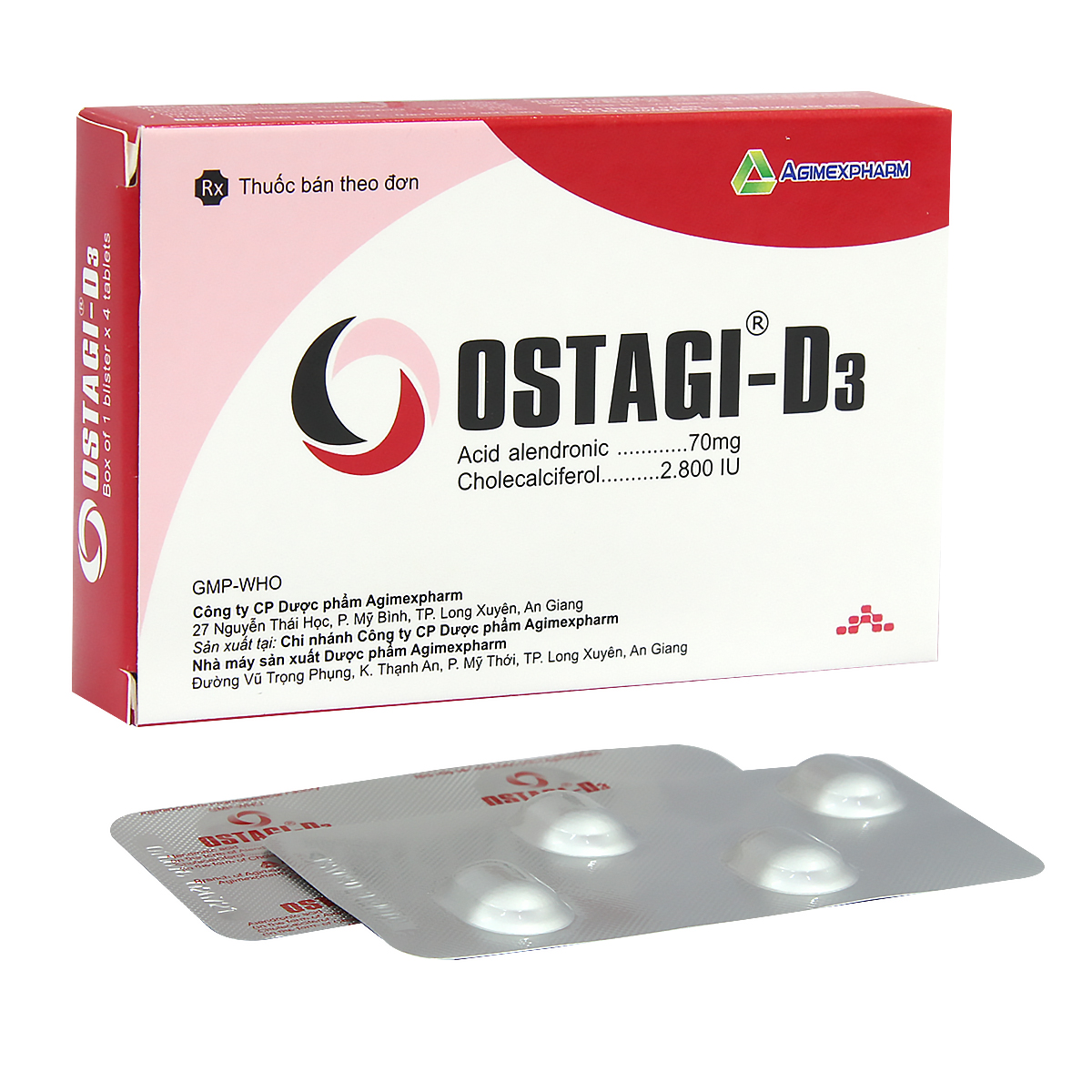
Chỉ định:
Điều trị loãng xương ở phụ nữ mãn kinh, điều trị loãng xương ở đàn ông. Thuốc làm tăng khối lượng xương và ngăn ngừa gãy xương, kể cả khớp háng, cổ tay và đốt sống (gãy do nén đốt sống) và bổ sung vitamin D cho cơ thể.
Dự phòng và điều trị loãng xương do dùng corticosteroid.
Điều trị bệnh xương Paget: Cho người bị bệnh Paget có phosphatase kiềm cao hơn ít nhất hai lần giới hạn trên của bình thường, hoặc người có triệu chứng bệnh, hoặc người có nguy cơ mắc biến chứng sau này do bệnh đó.
Liều dùng:
Liều khuyến cáo là uống mỗi tuần 1 viên duy nhất.
Để dễ nhớ, nên uống thuốc vào 1 ngày cố định trong tuần.
Khi quên uống liều hằng tuần, nên uống 1 viên vào sáng ngày đầu tiên khi nhớ ra. Không được uống 2 viên thuốc trong cùng một ngày, tiếp tục uống 1 viên/1 lần mỗi tuần vào ngày đã chọn từ trước.
Người cao tuổi:
Không cần thiết phải điều chỉnh liều ở người cao tuổi.
Người bệnh suy thận:
Không cần thiết phải điều chỉnh liều ở người bệnh suy thận từ nhẹ đến vừa (độ thanh thải creatinin từ 35 đến 60 ml/phút).
Không dùng thuốc này cho người bệnh suy thận nặng (độ thanh thải creatinin < 35 ml/phút) do chưa có kinh nghiệm lâm sàng.
Cách dùng:
Để thuốc dễ hấp thu, phải uống thuốc vào buổi sáng, ít nhất 30 phút trước khi ăn, uống hoặc dùng thuốc khác trong ngày. Các thức uống khác (kể cả nước khoáng), thức ăn và một số thuốc khác có thể làm giảm sự hấp thu của acid alendronic.
Phải uống trọn viên thuốc với một ly nước đầy (không ít hơn 200 ml), không được ngậm hoặc nhai nát viên thuốc. Bệnh nhân cần đứng hoặc ngồi thẳng trong ít nhất 30 phút sau khi uống thuốc, để thuốc vào dạ dày dễ dàng và giảm nguy cơ kích ứng gây tổn thương thực quản (viêm, loét, trợt, thủng). Không nên uống thuốc vào lúc đi ngủ.
Chống chỉ định:
Liên quan đến acid alendronic:
Dị dạng thực quản (ví dụ hẹp hoặc không giãn tâm vị) làm chậm tháo sạch thực quản.
Không có khả năng đứng hoặc ngồi thẳng trong ít nhất 30 phút, người có nguy cơ sặc khi uống thuốc.
Mắc bệnh ở đường tiêu hóa trên (khó nuốt, bệnh thực quản, viêm loét dạ dày tá tràng).
Quá mẫn với bisphosphonat hoặc với bất kỳ thành phần nào của thuốc.
Giảm calci huyết.
Suy thận nặng.
Liên quan đến cholecalciferol:
Tiền sử mẫn cảm với vitamin D.
Tăng calci máu do bất cứ nguyên nhân nào.
Sỏi thận kèm tăng calci niệu.
Cường cận giáp tiên phát.
Các trường hợp thận trọng khi dùng thuốc:
Các tình trạng cần thận trọng:
Liên quan đến alendronat:
Tác dụng phụ lên đường tiêu hóa trên:
Alendronat có thể gây kích ứng tại chỗ ở niêm mạc tiêu hóa trên. Cần thận trọng khi dùng alendronat cho bệnh nhân đang có các bệnh ở đường hô hấp trên, chẳng hạn như chứng khó nuốt, bệnh thực quản, viêm dạ dày, viêm tá tràng, loét, hoặc có tiền sử gần đây (trong vòng năm trước đó) các bệnh đường tiêu hóa quan trọng như loét dạ dày tá tràng, hoặc chảy máu tiêu hóa tiến triển, hoặc phẫu thuật đường tiêu hóa trên không phải là thủ thuật tạo hình môn vị.
Đã có báo cáo về những phản ứng có hại ở thực quản (đôi khi nghiêm trọng và phải nhập viện), như viêm thực quản, loét thực quản và trợt thực quản, hiếm khi dẫn tới hẹp hoặc thủng thực quản ở người bệnh điều trị bằng alendronat. Một số trường hợp nặng và cần nằm viện. Vì thế, cần cảnh giác với bất kỳ dấu hiệu hoặc triệu chứng nào báo hiệu về phản ứng thực quản và người bệnh phải được hướng dẫn để ngừng uống OSTAGI-D3 và báo cho bác sỹ ngay nếu thấy khó nuốt, đau khi nuốt, đau sau xương ức, ợ nóng mới xuất hiện hoặc nặng hơn.
Nguy cơ mắc tai biến nặng về thực quản gặp nhiều hơn ở những người bệnh nằm ngay sau khi uống thuốc hoặc không uống thuốc với cốc nước đầy và/hoặc vẫn tiếp tục dùng OSTAGI-D3 sau khi có những triệu chứng nghi là kích ứng thực quản. Vì vậy, cung cấp đầy đủ thông tin cho người bệnh để họ hiểu rõ các chỉ dẫn về liều lượng, cách dùng là rất quan trọng.
Mặc dù không thấy tăng nguy cơ trong những thử nghiệm lâm sàng mở rộng, đã có một số báo cáo hiếm gặp (sau khi đưa thuốc ra thị trường) về loét dạ dày và tá tràng, trong đó một số trường hợp nặng và có biến chứng.
Hoại tử xương hàm:
Hoại tử xương hàm thường liên quan đến nhổ răng và/hoặc nhiễm khuẩn tại chỗ (kể cả viêm xương tủy) đã được báo cáo ở các bệnh nhân ung thư đang được điều trị với phác đồ gồm có bisphosphonat tiêm tĩnh mạch. Nhiều người trong số những bệnh nhân này cũng đang được điều trị theo hóa trị liệu và corticosteroid. Hoại tử xương hàm cũng đã được báo cáo ở bệnh nhân loãng xương đang uống bisphosphonat.
Các yếu tố nguy cơ sau đây cần được xem xét khi đánh giá nguy cơ của từng cá nhân đối với xảy ra hoại tử xương hàm:
Hiệu lực của bisphosphonat (cao nhất đối với acid zoledronic), đường dùng và liều tích lũy.
Ung thư, hóa trị, xạ trị, corticosteroid, chất ức chế tạo mạch, hút thuốc.
Lịch sử bệnh về răng, vệ sinh răng miệng kém, bệnh nha chu, thủ thuật răng xâm lấn và hàm răng giả phù hợp kém.
Khám răng với nha khoa dự phòng thích hợp cần được xem xét trước khi điều trị với thuốc uống bisphosphonat ở những bệnh nhân có tình trạng răng xấu.
Trong khi điều trị, nếu có thể, những bệnh nhân này nên tránh thủ thuật nha khoa xâm lấn. Đối với bệnh nhân bị hoại tử xương hàm trong khi điều trị với bisphosphonat, phẫu thuật nha khoa có thể làm trầm trọng thêm tình trạng này. Đối với bệnh nhân cần phải thực hiện thủ thuật nha khoa, không có dữ liệu cho thấy việc ngưng dùng bisphosphonat làm giảm nguy cơ hoại tử xương hàm.
Trong điều trị với bisphosphonat, tất cả bệnh nhân cần được khuyến khích duy trì vệ sinh răng miệng tốt, khám răng định kỳ, và báo cáo bất kỳ triệu chứng nào về miệng như di động của răng, đau hoặc sưng tấy.
Hoại tử xương ống tai ngoài:
Hoại tử xương ống tai ngoài do sử dụng các bisphosphonat đã được báo cáo, chủ yếu liên quan đến điều trị dài hạn. Các yếu tố nguy cơ có thể xảy ra cho hoại tử xương ống tai ngoài bao gồm sử dụng steroid và hoá trị và/hoặc các yếu tố nguy cơ tại chỗ như nhiễm trùng hoặc tổn thương. Cần xem xét khả năng hoại tử xương ống tai ngoài ở những bệnh nhân dùng bisphosphonat có triệu chứng ở tai như đau hoặc chảy mủ, hoặc nhiễm trùng tai mạn tính.
Đau cơ xương:
Đã có báo cáo về các trường hợp đau xương, khớp, và/hoặc cơ ở những bệnh nhân dùng bisphosphonat. Kinh nghiệm sau khi thuốc ra thị trường cho thấy các triệu chứng này hiếm khi nặng và/hoặc cản trở hoạt động bình thường. Thời gian khởi phát triệu chứng thay đổi từ một ngày đến vài tháng sau khi bắt đầu điều trị. Hầu hết bệnh nhân đều giảm triệu chứng sau khi ngừng điều trị. Một nhóm nhỏ bệnh nhân đã tái phát các triệu chứng khi dùng trở lại cùng một loại thuốc hoặc một bisphosphonat khác.
Gãy xương đùi không điển hình:
Gãy dưới mấu chuyển và thân xương đùi không điển hình đã được báo cáo với liệu pháp bisphosphonat, chủ yếu ở bệnh nhân được điều trị lâu dài vì loãng xương. Các vết gãy xương ngang hoặc chéo ngắn có thể xảy ra bất cứ nơi nào dọc theo xương đùi từ ngay dưới mấu chuyển nhỏ đến ngay phía trên lồi cầu. Gãy xương xảy ra sau khi bị chấn thương rất nhỏ hoặc không có chấn thương và một số bệnh nhân bị đau đùi hoặc háng, thường liên quan đến các đặc điểm hình ảnh của vết nứt trong xương, nhiều tuần đến nhiều tháng trước khi biểu hiện gãy xương đùi hoàn toàn. Gãy xương thường cả hai bên; do đó xương đùi phía bên kia nên được kiểm tra ở những bệnh nhân được điều trị với bisphosphonat đã gãy thân xương đùi. Khó chữa lành các trường hợp gãy xương cũng đã được báo cáo. Việc ngừng dùng liệu pháp bisphosphonat ở những bệnh nhân nghi ngờ bị gãy xương đùi không điển hình nên được xem xét trong khi chờ ước lượng tình trạng bệnh nhân, dựa trên đánh giá rủi ro lợi ích từng cá nhân.
Trong lúc điều trị bằng bisphosphonat nên khuyên bệnh nhân báo cáo bất kỳ cảm giác đau nào ở đùi, hông hoặc háng và bất kỳ bệnh nhân có các triệu chứng như vậy nên được đánh giá gãy xương đùi chưa hoàn toàn.
Suy thận:
Không nên dùng thuốc này cho người bệnh suy thận có độ thanh thải creatinin < 35 ml/phút.
Chuyển hóa xương và khoáng chất:
Do các tác dụng tích cực của alendronat trong việc làm tăng khoáng xương, giảm calci và phosphat trong huyết thanh có thể xảy ra đặc biệt ở bệnh nhân dùng glucocorticoid, sự hấp thu calci ở những người này có thể giảm. Các tình trạng này thường giảm nhẹ và không có triệu chứng. Tuy nhiên đã có báo cáo về các trường hợp hiếm bị hạ calci máu triệu chứng, đôi khi nghiêm trọng và thường xảy ra ở bệnh nhân trong tình trạng có khuynh hướng mắc bệnh (ví dụ: Giảm năng tuyến cận giáp, thiếu vitamin D và hấp thu kém calci).
Phải điều trị chứng giảm calci máu trước khi bắt đầu điều trị bằng alendronat. Cũng phải điều trị một cách hiệu quả các rối loạn khác về chuyển hóa vô cơ (ví dụ thiếu hụt vitamin D).
Phải chỉ dẫn người bệnh bổ sung calci nếu lượng hằng ngày trong khẩu phần ăn không đủ.
Thận trọng khi sử dụng cho những bệnh nhân suy thận hoặc sỏi thận, bệnh tim, xơ vữa động mạch, bệnh nhân bị tăng phosphat huyết nặng.
Liên quan đến cholecalciferol:
Vitamin D3 có thể làm gia tăng mức độ tăng calci huyết và/hoặc tăng calci niệu khi dùng cho người mắc các bệnh có liên quan tới sự sản xuất quá mức calcitriol mà không điều hòa được (ví dụ: Bệnh bạch cầu, u lympho bào, bệnh sarcoid). Những người bệnh này cần được theo dõi nồng độ calci trong nước tiểu và huyết thanh.
Bệnh nhân kém hấp thụ có thể không hấp thụ đầy đủ vitamin D3.
Tá dược:
Thuốc này có chứa lactose. Bệnh nhân mắc các rối loạn chuyển hóa di truyền hiếm gặp về không dung nạp galactose, chứng thiếu hụt lactase Lapp hoặc kém hấp thu glucose-galactose không nên sử dụng thuốc này.
Các khuyến cáo dùng thuốc cho phụ nữ có thai và cho con bú:
Thuốc này được chỉ định dùng cho phụ nữ mãn kinh, không dùng cho phụ nữ có thai hoặc phụ nữ đang cho con bú.
Tác động của thuốc đến khả năng lái xe và vận hành máy móc:
Chưa có nghiên cứu nào về ảnh hưởng của thuốc trên người đang lái xe và vận hành máy móc nhưng nên thận trọng vì đã có báo cáo tác dụng phụ tuy hiếm gặp là ảo thính giác, rối loạn thị giác.
Tương tác của thuốc với các thuốc khác và các loại tương tác khác:
Liên quan đến alendronat:
Estrogen: An toàn và hiệu quả của việc sử dụng đồng thời liệu pháp thay thế hormon và alendronat cho phụ nữ sau mãn kinh chưa được xác định, vì vậy khuyến cáo không nên dùng đồng thời.
Sữa, các chất bổ sung calci, magnesi hoặc các thuốc chứa nhôm (chống acid): Có thể làm giảm hấp thu alendronat. Vì vậy người bệnh phải chờ ít nhất nửa giờ sau khi uống alendronat mới dùng bất kỳ thuốc nào khác.
Ranitidin tiêm tĩnh mạch làm tăng sinh khả dụng alendronat đường uống.
Thuốc chống viêm không steroid (NSAID): Dùng phối hợp với alendronat có thể tăng nguy cơ loét dạ dày, nên phải thận trọng khi phối hợp.
Sắt: Thuốc uống có sắt làm giảm hấp thu alendronat.
Kháng sinh aminoglycosid: Tăng nguy cơ giảm calci huyết nếu được dùng đồng thời.
Liên quan đến cholecalciferol:
Không nên dùng đồng thời với các glycosid trợ tim vì độc tính của glycosid trợ tim tăng do tăng calci huyết, dẫn đến loạn nhịp tim.
Không nên dùng đồng thời với corticosteroid vì corticosteroid làm cản trở tác dụng của vitamin D.
Dùng đồng thời với cholestyramin, colestipol hydroclorid có thể làm giảm hấp thu vitamin D qua đường tiêu hóa.
Dùng đồng thời với thuốc lợi niệu thiazid cho những người suy cận giáp có thể dẫn đến tăng calci huyết.
Dùng đồng thời với phenobarbital, phenytoin có thể làm giảm nồng độ các chất chuyển hóa của vitamin D trong huyết tương và tăng chuyển hóa vitamin D thành những chất không có hoạt tính.
Tác dụng không mong muốn:
Các phản ứng có hại của thuốc chủ yếu là phản ứng của alendronat, các phản ứng này thường nhẹ và nói chung không cần phải ngừng thuốc, thường gặp là tác dụng không mong muốn ở đường tiêu hóa.
Thường gặp, ADR > 1/100:
Hệ thần kinh trung ương: Nhức đầu (2,6%), đau (4,1%).
Tiêu hóa: Đầy hơi (2,6%), trào ngược acid (2%), viêm loét thực quản (1,5%), nuốt khó, chướng bụng (1%), tiêu chảy.
Ít gặp, 1/1000 < ADR < 1/100:
Da: Ban, ban đỏ (hiếm).
Tiêu hóa: Viêm dạ dày (0,5%).
Hiếm gặp, ADR < 1/1000:
Dị ứng với alendronat nói riêng và bisphosphonat nói chung.
Ảo thính giác, rối loạn thị giác. Hoại tử xương hàm, hư khớp hàm. Có thể gãy xương đùi khi dùng thuốc kéo dài.
Quá liều và cách xử trí:
Các triệu chứng quá liều:
Giảm calci máu, giảm phosphat máu, và các phản ứng không mong muốn ở đường tiêu hóa trên như rối loạn tiêu hóa ở dạ dày, ợ nóng, viêm thực quản, viêm hoặc loét dạ dày có thể do uống quá liều alendronat.
Cách xử trí:
Không có thông tin riêng biệt về điều trị quá liều alendronat.
Nên cho dùng sữa và các chất kháng acid để liên kết alendronat. Do có nguy cơ kích ứng thực quản, không được gây nôn và người bệnh vẫn phải ngồi thẳng đứng. Thẩm tách không có hiệu quả.
Các đặc tính dược lực học:
Liên quan đến alendronat:
Alendronat là một aminobisphosphonat tổng hợp, một chất đồng đẳng của pyrophosphat, có tác dụng đặc hiệu ức chế tiêu xương. Khác với pyrophosphat nhưng giống etidronat và pamidronat, alendronat không bị các phosphatase thủy phân. Các nghiên cứu tiền lâm sàng cho thấy alendronat tích tụ chọn lọc ở các vị trí tiêu xương đang hoạt động, nơi mà alendronat ức chế sự hoạt động của các hủy cốt bào. Alendronat gắn vào xương và có nửa đời đào thải cuối cùng kéo dài tới trên 10 năm; tuy nhiên alendronat vẫn có hoạt tính dược lý khi gắn vào khung xương. Các nghiên cứu lâm sàng cho thấy điều trị bằng alendronat có thể làm tăng đáng kể khối lượng xương ở xương cột sống, cổ xương đùi và mấu chuyển.
Trong các nghiên cứu lâm sàng ở phụ nữ mãn kinh từ 40 đến 85 tuổi bị loãng xương (được xác định là có khối lượng xương thấp, ít nhất là 2 độ lệch chuẩn dưới trung bình của thời kỳ trước mãn kinh), điều trị bằng alendronat làm giảm đáng kể số lần gãy đốt sống sau 3 năm dùng thuốc. Mật độ chất khoáng ở xương tăng rõ sau 3 tháng điều trị bằng alendronat và còn tiếp tục trong suốt quá trình dùng thuốc. Tuy nhiên sau 1 – 2 năm điều trị, nếu ngừng liệu pháp alendronat thì không duy trì được sự tăng khối lượng xương. Điều đó chứng tỏ phải liên tục điều trị hàng ngày mới duy trì được hiệu quả chữa bệnh.
Liên quan đến cholecalciferol (vitamin D3):
Cholecalciferol (vitamin D3) có tác dụng duy trì nồng độ calci và phospho bình thường trong huyết thanh bằng cách tăng hấp thu các chất khoáng này từ thức ăn ở ruột non. Các dạng hoạt hóa của cholecalciferol huy động calci từ xương vào máu và đẩy mạnh tái hấp thu phosphat ở ống thận và tác động trực tiếp lên các tế bào tạo xương để kích thích phát triển xương. Các dạng hoạt hóa của cholecalciferol có tác dụng ức chế ngược đối với sự tạo thành hormon cận giáp (PTH).
Các đặc tính dược động học:
Liên quan đến alendronat:
Alendronat được hấp thu ít theo đường uống. Hấp thu thuốc giảm bởi thức ăn, bởi các chất chứa calci hay các cation đa hóa trị. Khả dụng sinh học đường uống khoảng 0,4% nếu uống 30 phút trước khi ăn; và hầu như không đáng kể nếu uống trong vòng 2 giờ sau khi ăn. Khoảng 78% thuốc được hấp thu gắn với protein huyết tương. Thuốc không bị chuyển hóa; khoảng một nửa thuốc hấp thu được đào thải qua nước tiểu; nửa còn lại được giữ lại ở xương trong một thời gian dài.
Liên quan đến cholecalciferol (vitamin D3):
Cholecalciferol (vitamin D3) được hấp thu tốt qua đường tiêu hóa nếu hấp thu mỡ bình thường và được hấp thu từ ruột non.
Sau khi hấp thu cholecalciferol vào máu thông qua vi thể dưỡng chấp của bạch mạch và sau đó kết hợp chủ yếu với một alpha-globulin đặc biệt (protein gắn vitamin D). Các chất chuyển hóa (hydroxyl hóa) của cholecalciferol cũng tuần hoàn trong máu kết hợp cùng với alpha-globulin.
Cholecalciferol được hydroxyl hóa ở gan tạo thành 25-hydroxycholecalciferol sau đó chất này tiếp tục được hydroxyl hóa ở thận để tạo thành chất chuyển hóa có hoạt tính 1,25-dihydroxycholecalciferol và những dẫn chất 1,24,25-trihydroxy. Nửa đời của các chất chuyển hóa 25-hydroxycholecalciferol trong máu khoảng từ 10 ngày đến 3 tuần và nửa đời của các chất chuyển hóa 1,25-dihydroxycholecalciferol khoảng 4 – 6 giờ.
Vitamin D và các chất chuyển hóa của nó được đào thải chủ yếu qua mật và phân, chỉ có một lượng nhỏ xuất hiện trong nước tiểu.


 English
English